Iron, together with other trace metals, is an essential micronutrient, required for the growth of all organisms, including phytoplankton that form the base of the marine food web. Given that phytoplankton convert CO2 into biomass that partly settles into the deep ocean, trace metals are key players in global climate. However, some key ocean regions remain understudied and various crucial processes remain poorly understood, making informed-policy decisions on climate strategies and management rather challenging. One of these understudied regions is the High Latitude North Atlantic, where phytoplankton and thus primary productivity is limited by the availability of iron. Since the high latitudes are changing rapidly, scientist are urged to understand how both current and future ocean changes effect global biogeochemical cycles, notably the marine iron cycle.
Consequently, the MetalGate team will collect samples for trace metals, various isotopes, nutrients, phytoplankton, and sedimentological parameters during the expedition to unravel the role metals play in this climate relevant region. The scientists will also conduct controlled bio-assays at ecologically relevant conditions to improve current understanding of the linkages between phytoplankton physiology and biogeochemistry in the high latitude North Atlantic. Samples will also be collected for colleagues from the USA, UK, Spain, Switzerland, and Brazil that cannot join the cruise, making this expedition a truly interdisciplinary and international endeavour. Insights from this work will enhance the understanding of local biogeochemical cycles and their connections to global cycles and thus climate. Such data will also result in better predictions of the likely impact, both now and in the future, of imminent Arctic and sub-Artic climate change on ocean health.
Wednesday 21 July 2021
The MetalGate Team is currently sampling their 3rd out of 40 stations during their 4 week long expedition in the North Atlantic. The team is set to sample the water column and sediments for trace metals, isotopes, ligands, and biological parameters, that is, phytoplankton in the surface ocean. Instruments are working, spirits are high, and the weather is good.

|
|
|
Tape on tape that is the only wayQuote of the day by Loay Jabre |
Saturday 24 July 2021
By Anna Koek
When I was working with Willem on a project using a dataset from the Antarctic Peninsula almost 18 months ago, he at some point asked me if I would be in for some fieldwork experience. Without knowing what he was talking about, I said yes immediately! Of course I didn’t know that despite a slight delay due to some global pandemic, missing a plane and bags staying behind in Amsterdam, I would be cruising around Iceland on the Pelagia, investigating iron limitation in the Atlantic ocean this summer.
My first week ever on a research vessel has been amazing! We were escorted out of the port of Reykjavik by a couple of puffins and a fin whale, sampled 5 different stations, started and measured our first iron addition experiment and we were very lucky with the weather. As the biological team, we are only interested in the top layer of the ocean, where most biological activity takes place. That means most of the time we are waiting until the CTD has travelled 2000m to the seafloor and all the way up to the surface again, where we sample the top 50m for marine microalgae. In the mean time we help out the other teams, or, as they are very organized and usually don’t need much help, we sit in the sun and enjoy the view on the ocean and the occasional sighting of a pod of pilot whales. In about 4 weeks, when we set foot on land again, we hope to have gathered a lot of data that can tell us why marine microalgae are not reaching there full potential in the high latitude North Atlantic and what the role of iron in these waters is!

Saturday 24 July
By Rebecca Zitoun
We had a few hiccups along the way since the last blog post, starting at station 3 with a malfunctioning trace metal clean CTD (i.e. the custom built UCC from NIOZ). Luckily, we had all the necessary spare parts on board, thanks to NMF, and within hours, our all-rounder chief scientist, Rob Middag, together with the Pelagia crew, got the instrument working again. We had to make up for the lost time though by skipping station 4 and 5, but on the bright side the instrument is working very smoothly now and all the scientists had heaps of time to organise their workspace and prepare for station 6. We even had time to start the first of our five bioassays during transect while testing the UCC in deep water. The bioassays will look at the effect of iron addition, ammonium addition, and increased temperatures on prevalent phytoplankton. The bioassay will run for the next 4 days and the results will give us insights on how climate change will impact phytoplankton biomass and composition in the High latitude North Atlantic.

We also just finished the second successful deployment of our custom built Bottom-Gradient- Sampler (BGS). The BGS was built to sample the water column close to the seabed that can’t be reached by the CTD rosette. Hence, the collected water samples will give us a better understanding of the fate and cycling of trace metals in the lowest water column, close to the sediment.

We are now on transit to station 7 for another deployment of the UCC.
Video showing the BGS (bottom gradient sampler)
|
|
|
If it doesn't work, try and try again and if it still doesn't work, fix it, fix it and fix it.Quote of the day by Sharyn Ossebaar |

Sunday, 1st August 2021
by Loay Jabre – PhD Candidate, Hugh C. Morris Fellow
After 15 days, 600+ samples collected, 290 flights of stairs climbed, 610 liters of sea water passed through filters, and four lost sharpies, I am a bit tired, but my excitement for what’s ahead keeps increasing with every station and with every experiment we conduct. Yesterday I was energized even more by blue sunny skies, calm waters, and a majestic view of Greenland with massive sharp mountains in the distance and some glaciers emptying into the ocean. What a sight! I can’t wait for us to get closer to Greenland to sample Station 21.
We also just harvested our first short-term bioassay, where we incubated a phytoplankton community under different iron and nitrogen conditions. As always, we lined up like ants to carry water containers from the top deck down to the trace metal lab to be sampled. The day was filled with filtrations and small side experiments to help us better understand how phytoplankton growth is influenced by iron and nitrogen availability in the region.
I am joined on this cruise by my lab mate Lena Beckley. Lena is a master’s student in the same lab where I’m doing my PhD (Bertrand Lab, Dalhousie University) and is collecting data for her master’s thesis. She and I have been primarily focused on collecting samples for subsequent proteomics measurements, and have been busy performing several nutrient uptake experiments at different stations. (Lena also single handedly labeled almost all the 600+ samples we collected so far!)
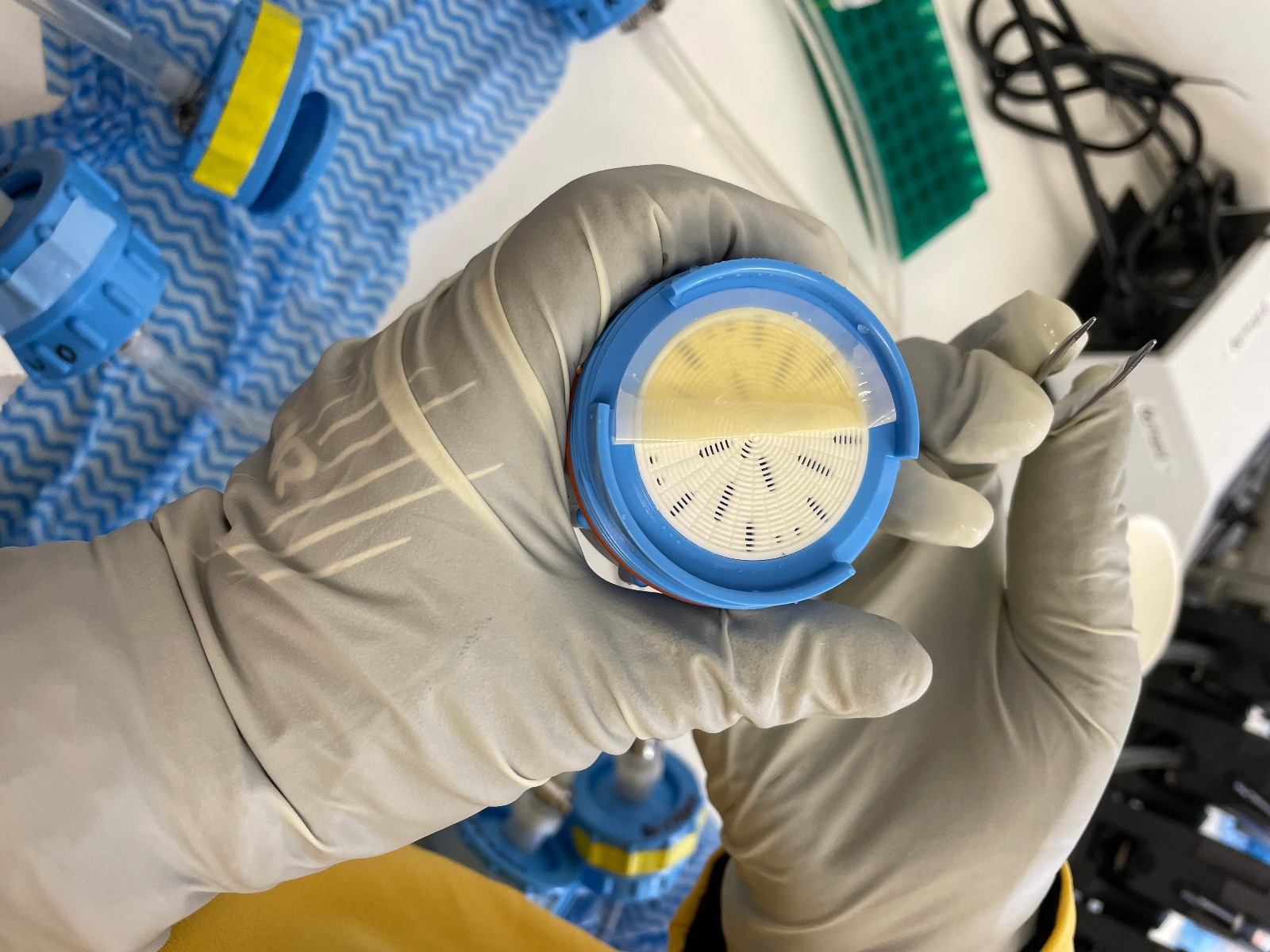
Proteomics: We are filtering water collected at various stations from different depths, as well as water from our bioassays, to capture tiny phytoplankton (the filter looks like a tiny phytoplankton pancake when it has sample on it). We’ll then take these phytoplankton samples back to the lab where we will extract proteins from the cells and analyze them using mass spectrometry. These measurements will give us clues about who was in the water (what kind of phytoplankton were there?) and what were they doing at the time we sampled them (e.g. were they experiencing iron stress in the natural environment? did our warm bioassay affect their nitrogen uptake enzymes?).
Nutrient Uptake Experiments: We are conducting these experiments in collaboration Rachel Sipler’s group who unfortunately could not join us on this cruise. For these experiments, we are ‘spiking’ natural phytoplankton communities (that we are collecting along the way) with isotopically labelled nutrients, then harvesting the phytoplankton on filters after an incubation period. Rachel Sipler’s group will then analyze these samples to understand how fast the phytoplankton uptake nutrients into their cells, and whether this is affected by different environmental conditions.
I feel so privileged to be part of this research cruise and to be learning so much from everyone on board. It’s been a joy meeting and getting to know everyone – both scientists and crew. In the months to come, I am looking forward to combining our molecular measurements with other measurements taken on board to construct a clearer picture of phytoplankton dynamics in these regions.

Monday, 2 August 2021
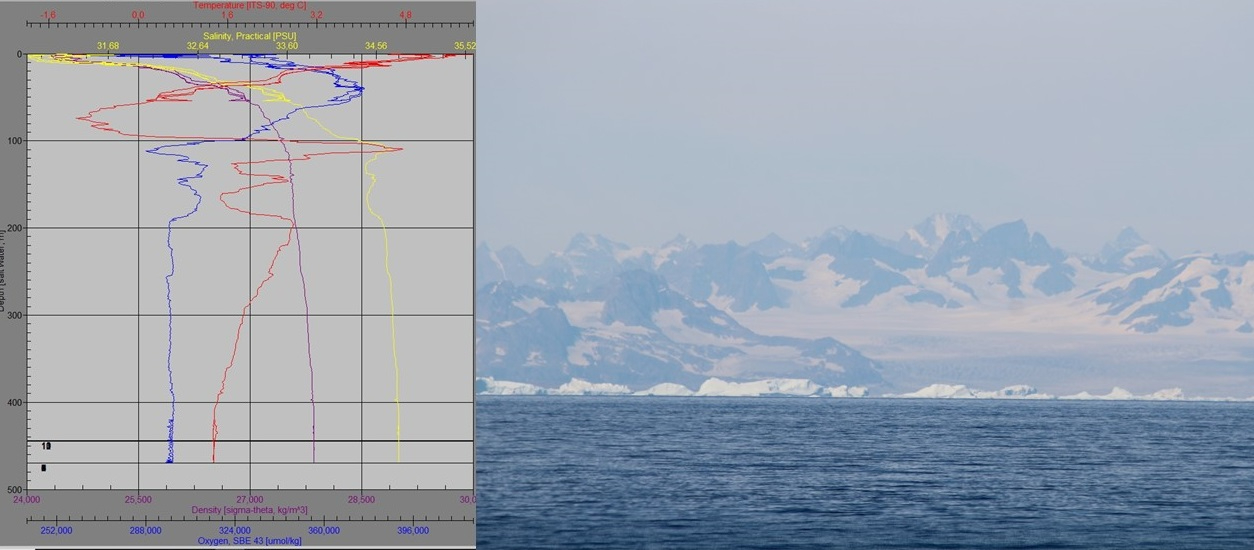
The MetalGate team sampled station 16 a couple of days ago, one of the closest stations to Greenland. At the same time, Greenland experienced its most significant melting event of the year as temperatures in the Arctic surged. We did see a drop in surface water salinity to 31 PSU with the CTD and this low salinity might have been a result of this melting event.
Friday, 6 August 2021
by Willem van den Poll
More than half way in the Metal gate cruise. The past weeks have been a rollercoaster of events. The chaos of Schiphol made place for the world onboard RV Pelagia. Getting used to the 24 h working routine around the CTD casts and the dull moments in between. Lining up in front of the CTD container to collect seawater for the numerous experiments that this cruise hosts. Hectic movement on deck during and after water collection. Trying to focus on following the experimental protocol in a moving container. Aiming to finish work in time to not to miss the excellent meals onboard. Meanwhile trying to adjust the experiments to the changing environmental conditions that we encounter. Working conditions early in the cruise below Iceland were bumpy and misty. This gave way for calm and sunny weather near the coast of Greenland, sampling during absolutely brilliant conditions, in a surreal landscape filled with fifty shades of blue and orange. Contrasts could not have been greater. An exciting encounter with a group of Orcas that crossed the path of the Pelagia just before passing the Arctic Circle.
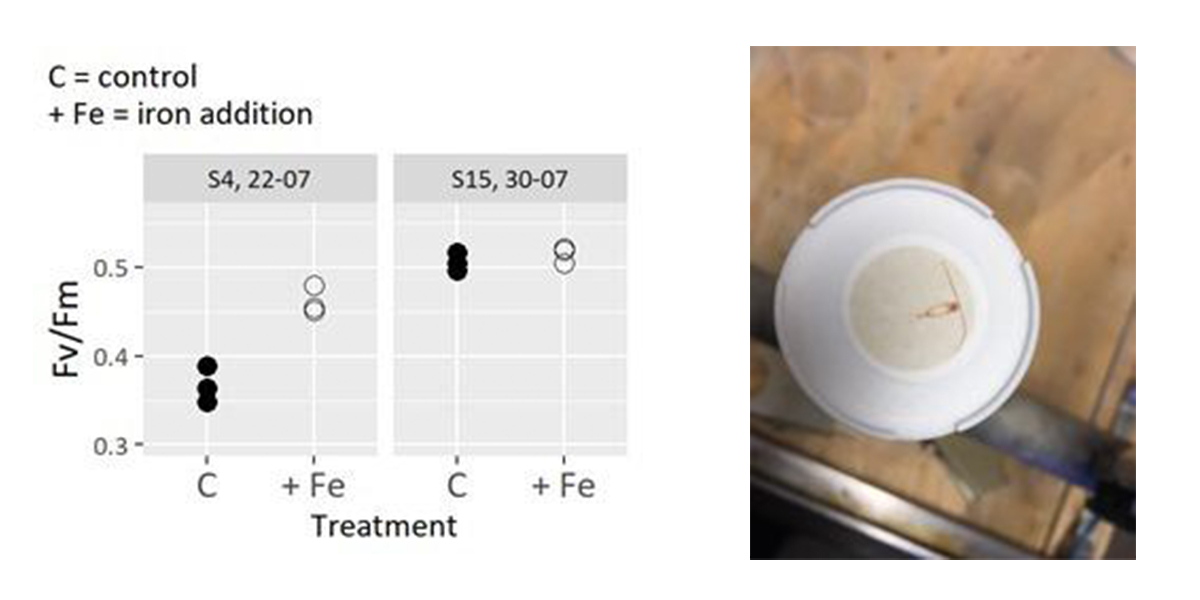
The sampled ocean region shows features of the Atlantic, the Arctic, and the melting Greenland glaciers. The low salinity surface layer that originates from the summer melt of Greenland was much more pronounced than I expected with salinity of 25 near the coast. The mixture of conditions is also reflected in the organisms that sometimes end up on our filters of which some most likely have an Arctic origin. We set of with a plan to investigate the photosynthetic performance of phytoplankton to the nutrient depleted summertime conditions. Cuun and me do numerous (20 so far) short bio assay experiments that target the conditions that limit phytoplankton growth in this region. We add dissolved iron to the sampled seawater and incubate it for 3 days and investigate changes in algal biomass and photosynthetic performance afterwards. Evidence for Iron limitation was most pronounced in the sub polar gyre. Further evidence for this unused productivity potential are the remaining nitrate concentrations in the surface ocean, up to 7 mM m-3 nitrate, meaning that half of the nutrients after deep winter mixing remain unused due to iron limitation. North of Iceland, the response to iron additions faded, and the nitrate surplus made way for nitrate limitation. Currently we are trading the cold East Greenland current for the warm Atlantic Gulf stream. Curious what we will encounter in the remaining part of the cruise. We are strapping our instruments to the ship in anticipation to a change in weather.
Monday, 9 August 2021
By Amber Annett
The Western leg of the MetalGate expedition is the busiest for Team Mud (Rhiannon, Xiangming and myself). Our sampling focuses on understanding how the deep waters of the Arctic basin are influenced by sediments as they flow over the sill of the Denmark Strait and into the deep North Atlantic. The interaction between sediment and overlying waters dictates whether sediments are a source or sink for things like carbon, pollutants, or iron (iron is the one I’m most interested in). For carbon and pollutants like mercury, keeping them in sediments is a good thing. For iron, fluxes out of sediment is one of the main sources of this essential micronutrient to the ocean.
Bottom currents can stir up sediment, releasing some of these elements back into the water column. However, if currents are too strong, all the fine sediment can be scoured away downstream, leaving coarse, hard-packed sediment at the seafloor that won’t exchange much with the water column. This is certainly the case at some of our stations here. The water flowing south at the bottom of the Denmark Strait forms the world’s largest waterfall – an underwater waterfall, that is. And unsurprisingly this means very strong bottom currents and several stations where we couldn’t recover any samples with the multicorer. Luckily our cruise track takes us far downstream, as well as upstream into the Arctic source waters, so the sediments we collected from these more distant locations will still be able to tell us how the concentrations of iron change in bottom waters across this region.

My fellowship, funded by the Natural Environment Research Council in the UK, combines different approaches to understand how much iron is released from sediments, and where this iron gets transported to. First, we need to measure how much iron is in sediments, so we use the multicorer to bring up seafloor sediment. We extract the porewaters – all the water in between the grains of sediment – by sucking this water out through a filter and into a syringe. We’ll measure what’s present in both the liquid (dissolved) and the solid sediment left behind.
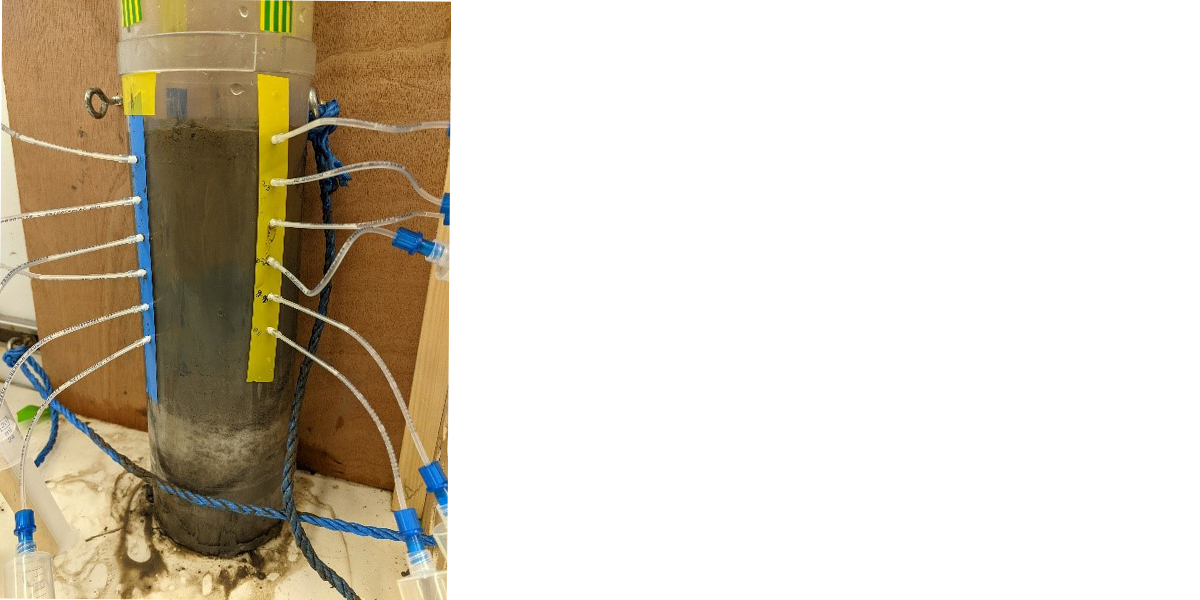
We also have a nifty way of measuring if (and how much) the sediments have been exchanging with the overlying waters. For this we use isotopes of my favourite element, Radium. Radium is naturally present in sediments, in extremely low (and therefore safe) amounts, because it is produced by the radioactive decay of thorium (which is also there naturally, at very low levels). We can tell how much radium there should be by measuring the thorium, and we can also measure how much radium is actually there. Usually we measure less than there should be, and this that the missing radium has been transferred into the overlying water. By combining this with our measurements of other elements (e.g. iron) we can also calculate how much iron is supplied from sediments to water.

Finally, after radium and iron are released from sediment into the water column, we follow this radium miles downstream. As radium decays at a known rate, the changes in concentration allow us to quantify how quickly any sediment-derived compounds are transported in the ocean interior. This part involves the heavy lifting, we need huge amounts of water to measure the very low abundances of radium. Luckily the rest of the science team as well as the crew of the Pelagia are always happy to help us move hundreds of kilograms of seawater from the CTD to the lab for processing - over 6000L of seawater processed so far! A big thanks to my Team Mud teammates, and to everyone helping us out!
Tuesday, 10 August 2021
By Xiangming Shi
Hello, I’m Xiangming. I’m from China and now working as a post-doctoral researcher in University of Connecticut in the US. I feel super lucky to participate in this cruise to explore the behavior of trace metals in the regions connecting the North Atlantic and the Arctic Oceans. My object in this cruise is to trace the benthic flux of Hg species with the 224Ra/228Th disequilibria in sediments, and to understand the biogeochemical cycling of Hg species, the inputs of Hg species to the deep ocean, and the sources and cycling of methylmercury (MeHg) in the subpolar North Atlantic. Little studies on benthic Hg fluxes have been done previously around this research area. We expect that 1) sediments are a net source of MeHg to the deep water and dominate the MeHg supply into the North Atlantic; 2) while sediments will be a net sink for inorganic Hg; and 3) the research region is a source for MeHg to the deep North Atlantic Ocean, primarily from shelf- and sediment-derived input.
In practice, we get the sediment with a multi-corer. For the 224Ra/228Th measurements, we slice one core at 1 cm interval and “cook” it to be a sediment “pancake”. The pancake is locked in a specific holder and determined in a Radium delayed coincidence counter (RaDeCC). For the benthic Hg flux quantification, we need to extract the porewater from the sediment core with Rhizons. Sometimes, only 2-3 mL porewater could be obtained from the deep layer for 2-hour extraction when the sediment is compact. Super precious samples!
Thank the NIOZ trace metal team for helping collect the water samples for Hg!

During this cruise, I’ve achieved many first-times. Now I’m at the most northern spot I’ve ever been on the earth. For the first time, I saw icebergs, wild killer whales and cute puffins. I painted my cup and had it compressed at the depth of 2000 m in Atlantic Ocean. I was told to dress up for a party during a cruise. I got a chance to threw out a buoy where I signed and drew for our “RaCE:TraX” team. A bunch of fun things! Luckily, we are enjoying the peaceful ocean with amazing sunrise and sunset every day! Thank the friendly crew and coworkers for their help on sampling and living on “Pelagia”!