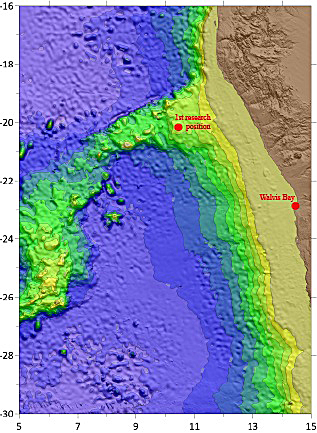
The region along the southwestern African coast is known for its low oxygen concentrations. The Benguela current enhances the upwelling of nutrient-rich deep waters which result in high surface productivity and consequently in low oxygen concentrations in shallow waters. However, there is a gradient from anoxic conditions in the south (Namibia) to hypoxic conditions towards the north (Angola). This gradient forms an excellent setting to research the ecological and biogeochemical functioning of eutrophic, oxygen-deficient marine systems which are important to understand with ongoing climate change. The aim of this interdisciplinary cruise is to identify the boundary where oxygen becomes the limiting environmental constraint along the margin. In collaboration with MARUM, we map the density and diversity of cold-water corals and associated fauna and measure the (near-bed) environmental conditions along the margins. In addition, we aim to measure the environmental conditions over a longer time for one year to determine if the low oxygen front shifts seasonally.
Another feature of this oxygen-depleted research area is the episodic/periodic occurrence of massive sulphur plumes, modern phosphorite formation, and strong modulation of microbial marine nitrogen cycling. Various research studies (e.g. IODP expedition Leg 175, Meteor cruise M48-2, M57-2/3, M76-2, M103, M122) were undertaken in the Namibian oxygen-depleted waters targeting these extreme events and providing a solid background. However, many open questions regarding the functioning of the Namibian oxygen-depleted waters remain. In close collaboration between the OCS and MMB department together with the UU and external partner MARUM, a couple of these questions are addressed in this cruise as well. Amongst others we aim to (1) reconstruct the historical intensity of the open-ocean oxygen-depleted zone over the last ~ 40 000 years, to (2) quantify the benthic exchange of essential redox elements such as iron, manganese, phosphorous, sulphur, and trace metals in relation to oxygen availability, and to (3) evaluate the impact on deoxygenation on the microbial ecology and carbon and nitrogen cycling.
We hope you'll enjoy reading our blog!
February 21th. We finally found them, the cold-water corals
In the morning of the 21th of February 2019, we were directly above the newfound cold-water coral province Kunene, named after the river entering the ocean at the Namibian and Angolan boarder. The Kunene province is at 240m water depth and lies in the oxygen minimum zone off the Namibia coast. Here we took a box-core and it was 11 o`clock when Jürgen knocked on my door saying that the first box-core was approaching the deck. Inside the core there were thousand fragments of dead cold-water corals rubble covered by olive black sandy mud sediments. After subsampling the box-core for analysis at NIOZ and Marum, we started sieving all the material. Among the corals (L. pertusa), there were polychaetes, sponges, sea pens (pennatulacea), ophiuroids, bivalves and gastropods as well as small shrimps and lobsters. We collected all the material for DNA and stable isotopic analyzes (d13C and d15N) in these organisms. After two hours, the second box-core, very similar to the previous one, arrived on the deck. The feeling was that it would be a long and tiring day of work. And it was.

A couple of hours later, we had to stop the sieving process because a 8.40m long (on-mound) piston core was drilled and positioned on deck. In an organized way, with each person was doing their task (e.g. labeling, cutting in 1m pieces and storage), we managed to process the core in less than an hour, when already the third box-core arrived on deck. The sieving process continued until the darkness of night, which prevented us from separating the polychaetes from the corals. Therefore, we took some time to rest, had dinner and played dart with friends. The sieving process of all 3 box-cores only ended next morning. And at that moment a new box-core from another area, was already on the deck waiting for us…
Rodrigo da Costa Portilho Ramos – Postdoc Marine Sedimentology at MARUM
February 25th | Nitrogen Cycling in the Benguela upwelling System
The Pelagia is sailing through the warm and shimmering waters of the Atlantic Ocean. We are near the Angolan-Namibian boarder, and for me this is day 31 on board of the Pelagia. The ocean seems to be full of life here. Birds and seals are flocking around the ship. However, it is actually the zones where life is limited, that interest us most during this expedition.
Ten days ago, we left the Namibian harbour at Walvis Bay to start the second leg of our sea research expedition. Here, a great underwater mount, called ‘Walvis Ridge’, rises up from the seabed, partially disrupting the cold Benguela current that is coming from the Antarctic.
During this expedition, we are studying the biogeochemical properties of the oxygen minimum zones (OMZs) present along the Namibian margin. More specifically though, I am studying the incomprehensible, utterly important, but invisible life of microorganisms involved in the nitrogen cycle of these anoxic waters.
Though a lack of oxygen has detrimental effects on bigger marine life, there are some microorganisms who thrive under these conditions. Every microbe has a role to play in earth’s biogeochemical cycles, and it’s the microbes that are involved in the nitrogen cycle that are our key actors. Within OMZs, a lot of bioavailable nitrogen is lost to the atmosphere as dinitrogen gas (N2) due to the metabolic activity of certain microorganisms (anammox bacteria and denitrifiers). This way, a disbalance might be created, where more nitrogen leaves the system than it enters. Indirectly, this also affects the carbon cycle, as nitrogen fuels phytoplankton growth in the ocean, which are key players in the uptake of carbon dioxide.
During this expedition, I am investigating the rate at which anammox bacteria and denitrifiers are producing N2 gas in the current OMZs, but I am also investigating the lipids they are producing. Lipids can be used as biomarkers, to trace back microorganisms of a specific taxonomic group or species in older sediments. So, when we know which microorganism produces what type of lipid (and what metabolic functions it performs), we can use this information to reconstruct the biogeochemical cycles of the past. This will hopefully provide us with a better idea of how these oxygen minimum zones will expand and/or contract in the future. To do so, I am incubating microorganisms from the water column and sediment with different nitrogen substrates, to promote their growth and subsequently look at the lipids they are producing.
Except for the micro-life that is surrounding us, we’ve also encountered some fascinating macro-fauna. Occasionally, a seal will pop along to give us a private performance of their washing ritual. But unfortunately, the biggest animals I’ve seen so far are the ones sitting next to me now. Talking of these animals, we have to get back to work now. But we’ll keep you updated!
Watery greetings,
Zoë van Kemenade - PhD student Marine Microbiology and Biogeochemistry department at NIOZ

February 16th | First sampling
After all preparations and logistics were completed in the port of Walvis Bay, we set sail in the evening of February 16. Our first destination was in the ‘arm pit’ from Walvis Ridge where we arrived in the next morning. Everyone was excited to start with the real work. After a good breakfast, which was prepared by our cook Iwan and his helper Vitalijs, we started with the first CTD.

CTD stands for conductivity, temperature and depth and it records several other oceanographic parameters we’re interested in to study the water column properties. The water depth at this station was about 1347m, so the CTD took a while until it reached the ocean floor. On its way back up to the surface we took water samples with the rosette from interesting depth we saw in the profile; for example, a depth with a high chlorophyll a or low oxygen concentrations. All water that we took was subsequently filtered for further analysis when we’re back at the NIOZ at our home laboratories.

In the evening, we took a piston core. The core is lowered in the water until it almost reaches the sea floor from where it falls into the soft sediments. Everyone hopes that it falls into the sediments as much as possible because with the recovered sediments it possible to get insights to the past climate. Everyone on board bet on the length, and Jürgen was the happy winner. His bet was closest to the 14.67m long core. The core was cut into 1m sections, on the picture below you can see our chief scientist in action – go Darci!
Laura Korte - department of Ocean Systems at NIOZ