
Introduction
Nanomaterials seem like something that humanity developed over a long process of industrialization and research. They have various industrial applications, from catalysts to coatings, drug delivery and many more. But nanomaterials were not invented by humanity, they have been critical natural components of the Earth since its formation and still play an important role in a plethora of geochemical and geological processes.
To this day, deep-sea hydrothermal vents, so-called black smokers, eject vast amounts of iron-containing nanoparticles into the ocean. Similar nanoparticles are known to catalyze the fixation of the greenhouse gas CO2 to hydrocarbons, a property that makes the hydrothermal particles a big potential player in the Earth’s carbon cycle and could unveil new ways for carbon fixation. Ultimately, such catalytic particles could have even played a crucial role in the emergence of life on Earth as hydrocarbons are one of the most central prerequisites for life as we know it.
To understand the past, current and future role of hydrothermal iron nanoparticles on the Earth’s biogeochemical system, UU and NIOZ experts are going on a research excursion to the Mid-Atlantic Ridge in August 2022. “We have built a truly exciting joint project between UU and NIOZ called I-NANO short for iron nanoparticles”, says excursion chief and geochemist Peter Kraal (NIOZ).“We are stepping into uncharted territory here.” The research crew, led by Kraal, Oliver Plümper (UU) and Martina Preiner (NIOZ/UU), consists of geochemists, biologists, geologists and chemists.
Read the blog here:
Day 1 | 19 August 2022
by Anna Cutmore (NIOZ)
And we’re off!
After a productive and hectic morning, we have now set sail to the Mid-Atlantic Ridge from Terceira Island, Azores…

After breakfast there was a lot of unpacking of the scientific equipment, preparation of the onboard laboratories, and loading of final provisions. For Su Ding and myself (Anna Cutmore), our preparation focused on the two insitu pumps which we are responsible for deploying at our two sites: Menez Gwen and Rainbow. Our initial prep included cleaning the pumps, charging the batteries, and setting up the computer programme to ensure that they are ready to insert the filters tomorrow before our first deployment!

The purpose of these pumps is to collect lipids from the water column at a range of depths at our two sites. The pump collects these lipids on specialised filters, which we will remove and return to the NIOZ laboratories where we will analyse their content and observe any changes with depth.

After dinner, we made the most of the calm, balmy conditions and sat back to watch for cetaceans in the vast expanse of ocean that lay before us. With the surface of Atlantic looking like a sheet of glass, we certainly had no difficulty spotting them! In the distance we could see dolphins jumping in and out of the water, and in no time, we had a group swimming alongside us- a school of Atlantic spotted dolphins (Stenella frontalis). Another 20 minutes passed and we had another, much larger group of dolphins swimming beside the ship- this time Common Dolphins (Delphinus delphis). And finally, we had an amazing encounter with three short-finned pilot whales (Globicephala macrorhynchus), who were much less energetic and sensibly avoided coming too close to the ship!

Day 2 | 20 August 2022
by Daphne Cuvelier (University of the Azores)
How do we visualise the deep sea?
Today, Menez Gwen (850m depth) gave us a first taste of the deep sea. It is our first stop along the Mid-Atlantic Ridge and we arrived on site at midday. We started with a reconnaissance of the area by visualising the ecosystem, in this case the deep-sea hydrothermal vents, its surroundings and its fauna as well as screening the deep-sea floor for suitable sampling sites. But how do we visualise the deep sea?

The Hopper tow-cam is an HD video platform specially designed to be operated on board of the RV Pelagia. It is lowered on starboard side of the ship and dropped down at a predefined station of interest to start a video transect, subsequent waypoints were selected and a heading was decided upon. It is called a tow-cam because it has no own propulsion but is towed behind the ship. For this, an excellent communication between bridge and tow-cam operators is established.
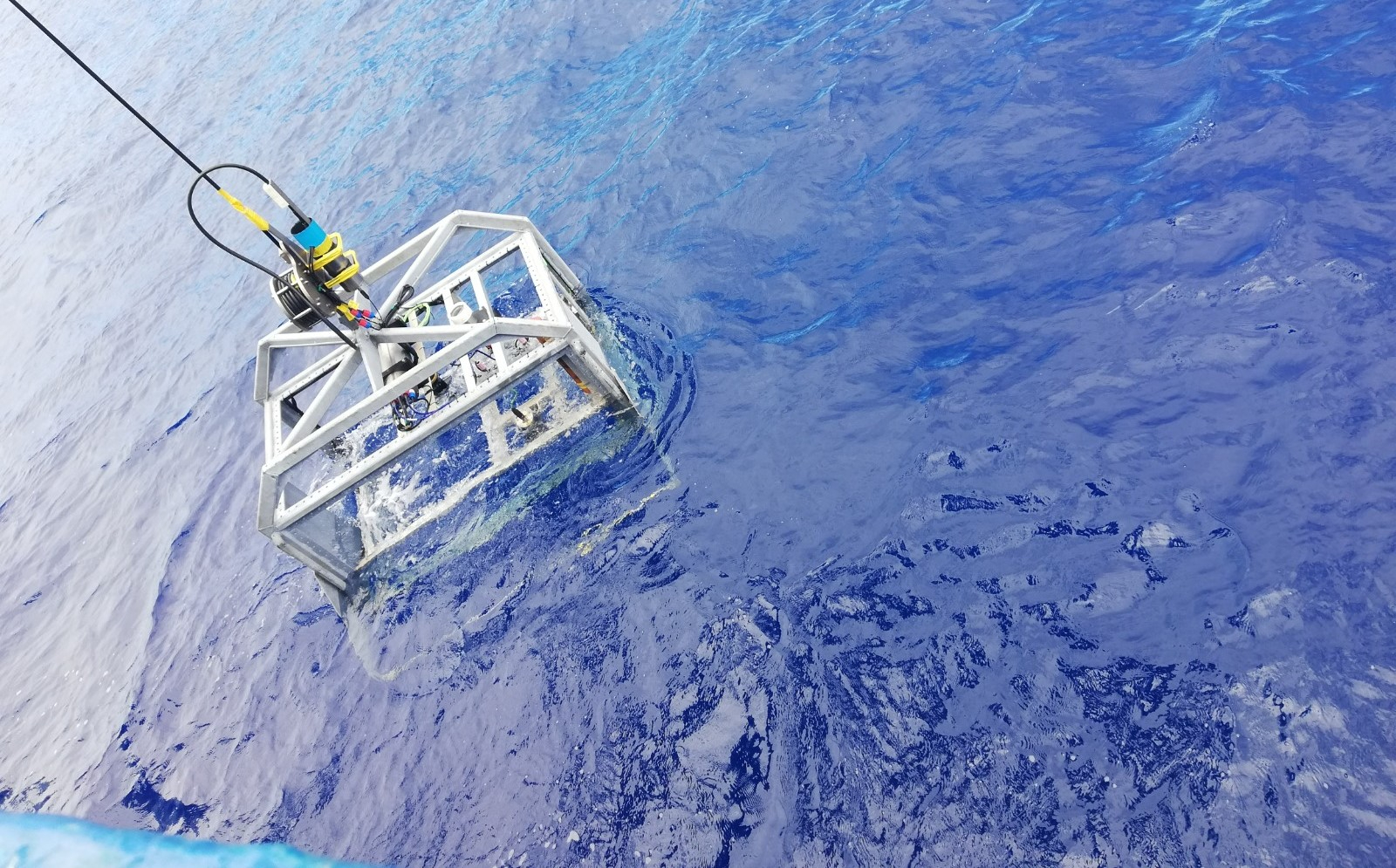
The Hopper is equipped with 3 cameras: one upward looking to check the connection with the cable to the ship, one forward looking for navigation and one downward looking at a slight angle to survey the seafloor. Furthermore, it has lights, lasers, sonar and a CTD. Lights are necessary to see in the dark deep sea and a CTD gives measurements on conductivity, temperature and depth. The sonar is used for object avoidance and to guarantee there are no collisions against for example changing topography features such as steep walls or hills. With a winch on deck, the tow-cam can be lowered (closer to the seafloor) or pulled in (tow cam is lifted up higher in the water column further from the seafloor) in order to avoid impacts. Finally, lasers are used for scale, to be able to estimate the size of organisms and geological structures.

Scientists on board watch and discuss in real-time the imagery collected by the Hopper!
Day 3 | 21 August 2022
by Guangnan Wu & Isabel van der Hoeven (NIOZ)
After the first days of getting used to being at sea, spotting some dolphins, collecting a picture of everyone’s happy face on board, labelling a lot of vials for sampling, and eating delicious food, the sediment team (Guangnan and Isabel) finally had real work to do…
The vent searching work yesterday resulted in some good luck in finding the desired spot for water sampling. Today, the CTD immediately landed on top of one of the largest vent areas at the Menez Gwen site. We could see the hot hydrothermal water coming out of the vent holes and there were mussels and some other animals (we are not biologists) around it. Unfortunately for the sediment team, there was barely any sediment on the seafloor of this “sea mountain”. As we saw on the camera, it consisted mostly of pillow basalts. Therefore, the sediment team did not fulfil its main task yet, but instead, sampled seawater at this site.

The idea of water column sampling is to use a normal CTD to sample inside the plume close to the vents, and the ultraclean-CTD in the water column above the plume. This way, we can cover the entire water column. Being sediment lovers, our passion also extends to the water above the sediment, because the extra salts and minerals expelled from the hydrothermal vents will end up on the sediment surface and get buried. Besides, the substances present in the water can be transported from sediment by for instance diffusion and resuspension. Thus, the water column samples will give us useful information for understanding the local biogeochemical cycles.

The CTD work went quite smoothly: we sub-sampled around 168 samples for nutrients and metals. We also prepared for the upcoming sediment work in a few days. In fact, for all the teams, today was a good preparation for when we arrive in Rainbow vent, the most important hydrothermal vent that we will visit.

It is now 9PM, 3 hours after sailing away from Menez Gwen. Evening activities include Mario Kart (racing on Rainbow road while on our way to Rainbow vent) and watching the Milky Way on the front deck. The journey of Cruise 64PE509 is still ongoing. Let’s see what Rainbow will bring us tomorrow.
Day 4 | 22 August 2022
by Lisa Eberhard, University of Utrecht & Andreas Beinlich, University of Bergen
In the morning we arrived at our second sampling site, the Rainbow vent field. A very first video survey of the ocean floor (the blog from day 2 explains in detail how we visualise the deep sea) gave as spectacular images of the ocean floor. We saw big chimneys releasing dense black smoke. These are the sites we are looking for! The smoke consists of fine-grained particles, which we want to characterise.
After the first video surveys and deciding for the best sampling location we lowered our CTD rosette to collect the smoky water. The CTD rosette has a frame with various sensors that monitor the conductivity, temperature and depth. Attached to the frame are also 24 bottles that can be filled with ocean water at different depth. In total, 288 litres of water can be collected and have to be filtered for the particles. For the filtering team the arrival of the CTD rosette back on board is the start of a long filtering marathon. Each bottle of the CTD holds about 12 litres of water. They have to be filtered litre for litre through our system.

Our onboard filter system to collect suspended particles works very well. Yesterday, we tested the system at Menez Gwen hydrothermal site and filtered about 230 litres of seawater. This was quite a busy day in the filter lab and is not a very effective way of cleaning the oceans. But at least we got a few particles.
Menez Gwen gave us few visible particles in each sample. But today we got particles! A lot of particles! The filters were completely covered with a black layer. We indeed sampled the smoke of the deep sea!
Day 5 | 23 August 2022
by Daphne Cuvelier (Institute of Marine Sciences, Okeanos of the University of the Azores in Horta)
Hey there, here is a little word from the resident biologist on board! Yesterday we did a video transect at the majestic Rainbow site (at 2300m depth) and we now completed our video transects for the I-NANO cruise, imaging the fascinating world of deep-sea hydrothermal vents and beyond. We used the video frame to help us localise and visualise the venting spots using the known coordinates from the previous cruises and scientific literature.
Hydrothermal vents are very peculiar ecosystems. They host a very specific fauna which cannot be found elsewhere. In the absence of sunlight and in the presence of high pressure and hot toxic, metal-laden fluids exiting the seafloor, the residing animals rely on chemosynthesis and chemosymbiosis for their energy provisions. The hydrothermal vent fauna on this part of the Mid-Atlantic Ridge consists of mussel beds and shrimp assemblages. During the video transects at Rainbow, we observed dead mussel beds (Fig. 1) as well as newly settled ones (Fig. 2) and tiny shrimps (Fig. 3).



We do not have the ability to sample the fauna from the deep sea during this cruise, but since the sites of interest are relatively well known, we can use the imagery collected here to study spatial and temporal variation of the fauna and investigate the relation and transition between active and inactive areas. At Rainbow, we crossed some active vent chimneys (Fig. 4) bellowing black smoke completely involving the video frame (Fig. 5), but also came across extinct chimneys (Fig. 6). Temporal variation studies will help us understand the natural dynamics of the faunal assemblages living in these parts of the deep sea, knowledge that is extremely important in an era in which the deep sea is no longer out of reach and is looked to for exploitation (e.g. fisheries, mineral extraction etc.).



Day 6 | 24 August 2022
by Oliver Plümper from the Department of Earth Sciences, Utrecht University, the Netherlands.
When scientists play with mud
Today some of us played with mud and found some amazing rock clasts. We used a massive box that we dropped onto the ocean floor to what we hoped would be sediment next to the hydrothermal vents. Unfortunately, for our sedimentologists on board, we went much deeper than anticipated and the entire box plus camera got covered in mud. Once back on board we decided to drop the mud onto the ship’s deck. Fortunately, for some of us hard-rock geologists, the mud was full of rock clasts that we happily fished out of the mud.


Turns out these rock clasts are serpentinites. A rock type that is critical to the origin of the hydrothermal vents. Serpentinites form when water, in this case likely a mixture of seawater and ‘magmatic water’, reacts with the minerals, olivine and pyroxene, of the upper mantle. The mid-Atlantic Ridge where we currently are is spreading so slow that mantle rocks are being brought up from great depth to the seafloor. Hence, as temperature fall below ~400 °C these rocks start to react with water. This process, generally referred to as serpentinization, results in the production of unique fluids which can range from pH 2 to pH 13. At Rainbow the pH is very low and massive amounts of iron are dissolved and expelled through the vents.

We were very thrilled to get some of these serpentinites on board to have a closer look. Now it is time to bring them to the Netherlands to cut them open and make very thin sections out of rocks. With these sections we can have a closer look at the mineralogy. These analyses will help us to gain insights into the evolution of the rocks below the Rainbow hydrothermal vents and how the unique geochemical environments came about. Stay tuned for some more fascinating science to come.

Day 7 | 25 August 2022
By Martina Preiner from both NIOZ (OCS) and Utrecht University (Department of Geosciences)
Reactors on a boat
On our i-NANO cruise, we not only wanted to collect fresh iron nanoparticles from the Rainbow hydrothermal vent field to see what we can do for (with) them (electron microscopy, organic extraction) – we wanted to know what they can do for us! Answering this question requires pressure reactors that ideally are employed in a stable, non-moving environment. But because we wanted to use the freshest material to see whether our nanoparticles are catalytically active (meaning if they can accelerate chemical reactions such as carbon fixation) we had to take them out of their natural habitat and make them seaworthy.
Not unlike myself, the reactors need a little extra help to perform their duty on a ship*. Although our conditions here on the Atlantic are admittedly considered mild to real seafarers, the long, slow waves typical for this time of the year can be a lot for untrained stomachs and reaction vessels. While I need the occasional sea sickness pill and a lot of cola, the reactors need wedges, chains, cables and anti-slip mats (so they require a lot more maintenance than I do, just saying…).


Once the reactors and stomach contents were staying where they are supposed to stay, we had to wait for the Rainbow vents – and the filtration team (s. blog day 4) – to provide us with fresh, black iron particles that were, after a quick round of cleaning, transferred in our reactors. In a final step, the reactors needed to be pressurized, so attached to gas bottles, flushed and filled with carbon dioxide and hydrogen gas. As this procedure has to be done outside of our lab container, without chains and wedges, the sea sickness pills and cola have to suffice to stabilize the scientist stabilizing the reactor.

While I am writing this, the Pelagia is already heading back to shore, a 46 hours journey, that the two gasses are hopefully using to react with each other with the help of our natural catalysts. But to find out if they really do, we’ll have to wait until the samples and we are back on shore. We might be able to bring reactors on a ship, but not a mass spectrometer. Or maybe next time.
*One could say, both the reactors and the reactor experimenters have to perform under pressure, but this is a lazy pun, I am better than that. Or am I?
