Sunday evening, 12th February 2023 - Matthew Humphreys (NIOZ), chief scientist
As often happens in ocean-going research, not all has gone to plan. Problems with our research vessel Pelagia’s engine delayed the start of the expedition by about 3 weeks, making the original plan of a round trip from Cape Town impossible to fit in with the ship’s later schedule. Instead, we are now en route from Cape Town to Cape Verde, heading north instead of south, and we are down to 7 scientists on board from an original complement of 12. This has been an enormous challenge and I’ve lost count of how many times I’ve had to go back to the drawing board with the expedition’s science plan. But despite these setbacks, we are all still very motivated and we are determined to draw the most science possible out of the expedition. We’re also very grateful to all the engineers, Pelagia’s officers and crew, and people behind the scenes at NIOZ who worked so hard to get the ship back up and running.

We departed from Cape Town yesterday (Saturday) morning and this afternoon paused to do a ‘dress rehearsal’ for the seawater sampling and ring nets. This was an important opportunity for everyone to get the hang of things because tomorrow we arrive at where we’re going to deploy the mooring and do the bulk of the scientific activities for the whole expedition – a ‘superstation’ with near-continuous work of one sort or another for over 30 hours. Once that has been completed, we’re looking forward to falling into a less exhausting routine for the rest of the transect!

Each member of the science team will write a blog post at some point over the coming three weeks on their work and experience at sea. We hope you enjoy following along!


Thursday 16 February, by Ben Cala
"And I’m gonna work 24 hours and I’m gonna work 24 more"
(to the tune of 500 miles by The Proclaimers and of course while adhering to safe work practices, such as regular breaks).

Superstation time! For most of us the most crucial time on the ship, with almost two days of continuous science experiments. Water samples were taken, a drifting sediment trap was set on the loose and recovered again a day later, and multinets were cast day and night to collect tiny marine organisms for later study. While others were more concerned getting things out of the water, for me the opposite was true: I had a mooring to deploy. In my PhD I am trying to understand what makes calcium carbonate dissolve. Think of making syrup: when no sugar is dissolved in the water, it is easy to dissolve it but the more is added the harder it becomes until it reaches a stage where the water is oversaturated and no more sugar dissolves. The same principles apply to the dissolution of calcium carbonates. At least for the most part. Curiously, there is data to show that calcium carbonates are also dissolving when the water is oversaturated, and I am trying to understand why that is. And what better way to do this than to chuck some of that calcium carbonate in water with different properties, wait for a while, and then see how much it dissolved?

Over the past months, I prepared many samples (960 in total, to be exact) of different types of calcium carbonates such as skeletons/shells of tiny marine organisms (foraminifera, pteropods) and corals. The superstation was the point where I attached them in regular intervals to a cable of 4.2 km length that would then reach from the bottom of the seafloor at 5000 m to 800 m below the surface.

I don’t know what I expected originally but I was surprised to see how many people were involved in the deployment of a mooring and how much work it was ultimately. Six members of the crew were with us at the back of the ship, keeping track of the cable and making sure it is in a safe place for us to attach our samples and then of course more people where needed to steer the ship or look after the engines. The whole process took 6 hours after which I was exhausted and took a several hour break while the crew were ready for other experiments half an hour later. Insane.


While science on a ship is certainly more stressful because time is valuable and limited, I find it also more wholesome: we are all working on something together, helping each other out whenever we can, and get excited about each other’s research projects. Apart from the crew and the scientists on board, I also want to give special thanks to the good weather and the amazing looking clouds which make working outside a joy, and finally to Bob Ross for his painting tutorial videos that help us relax at the end of a day’s work.

Monday 20th February , by Anne Kruijt
Our first week on the RV Pelagia – in illustrations.


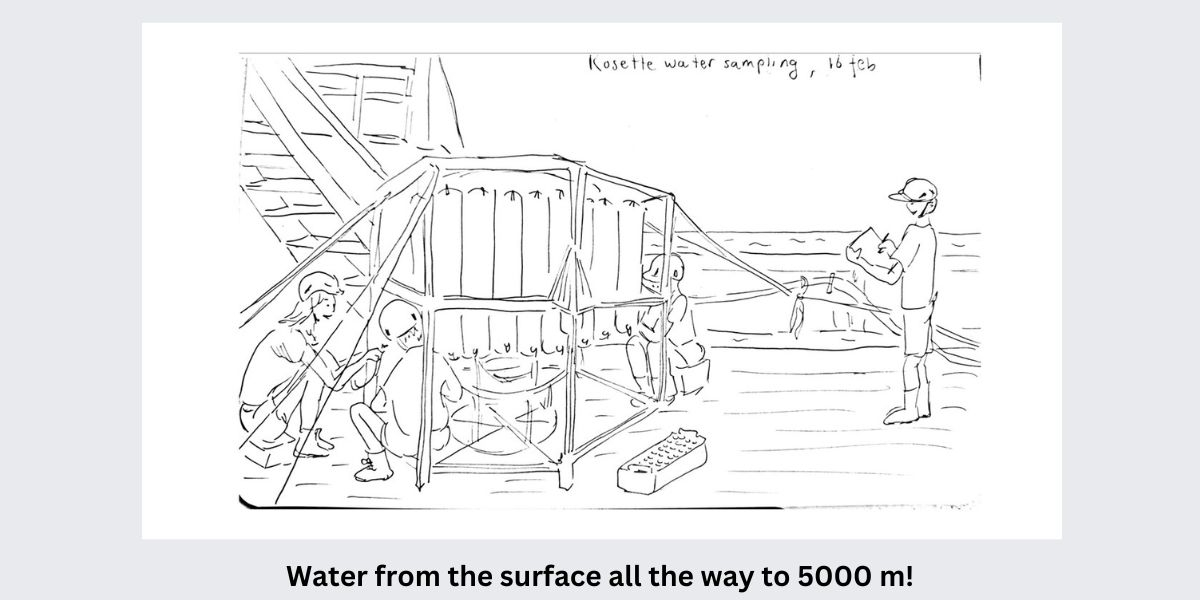

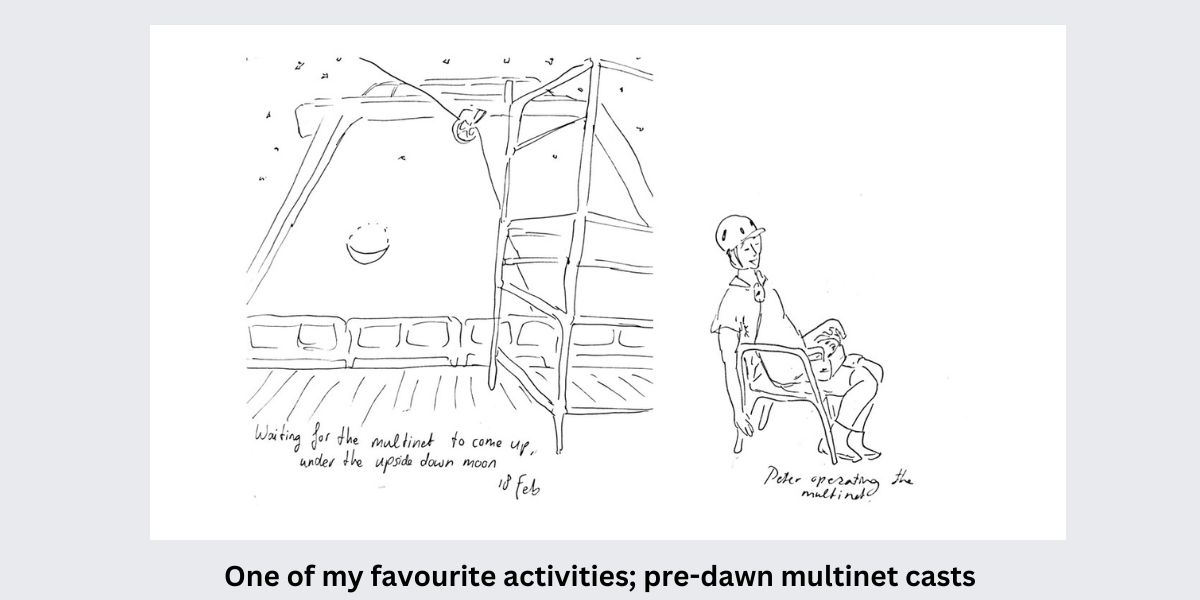


All illustrations by Anne Kruijt, PhD student at Utrecht University
Thursday 23 February 2023, by Robin van Dijk
Tales of a lone Marine Biologist
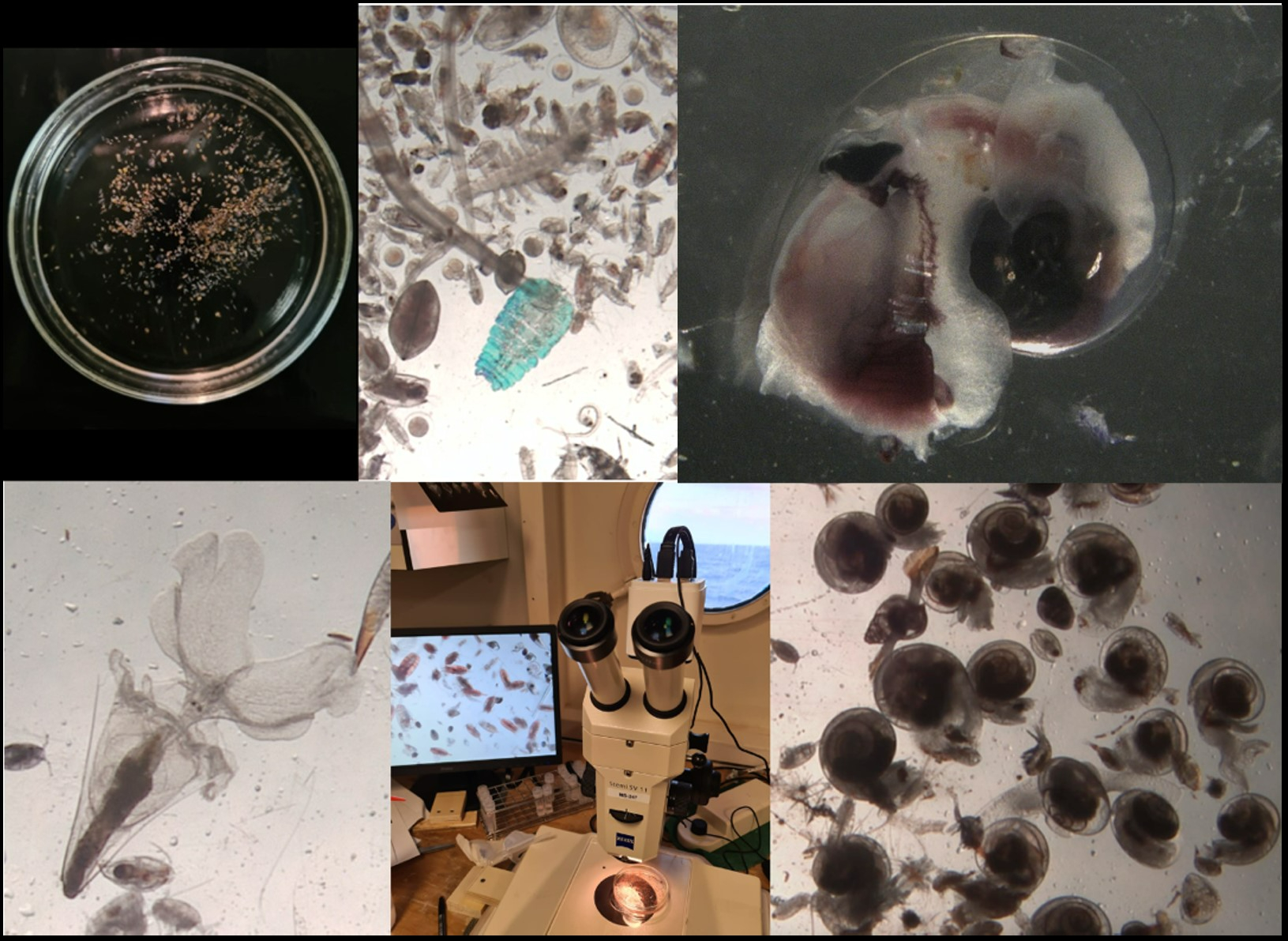
We are halfway the route of our expedition, and I feel like our science team is becoming more and more like a well-oiled machine. Sampling is running smooth, and we are becoming more efficient with processing the samples every day. Within the BEYΩND team, we have the plankton team on board that tries to understand the animal behind the mineral: the shelled pteropods. Although they are little, they are mighty. They are food for many other animals and play a large role in the Earth’s carbon cycle. But they are threatened: pteropods are predicted to be one of the first organisms to be affected by anthropogenic ocean acidification.

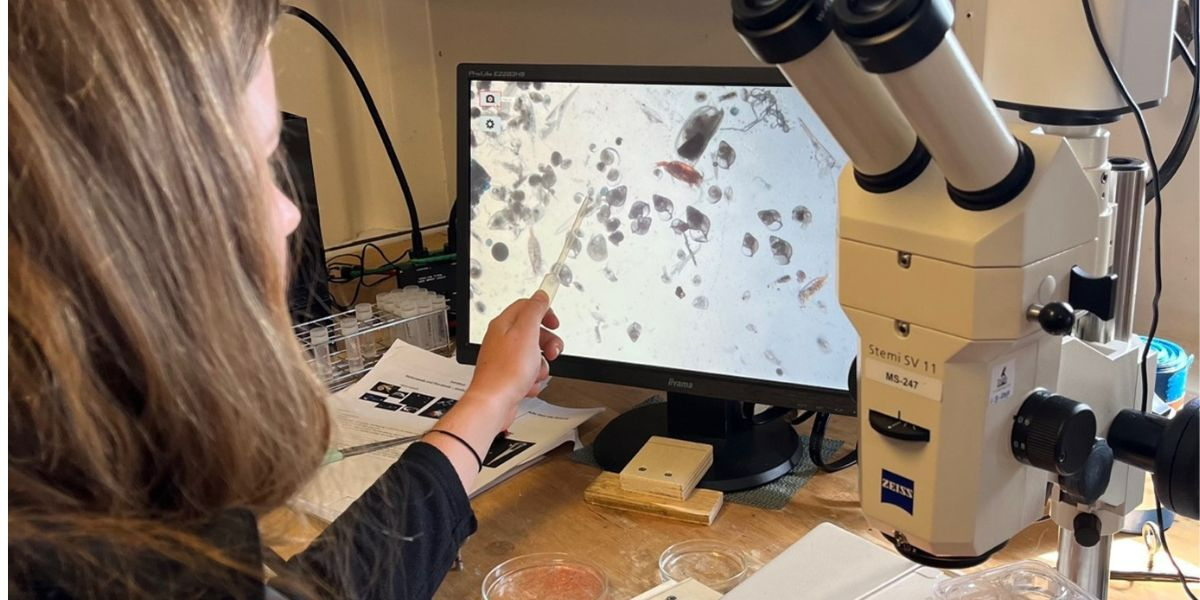
During this expedition we are taking samples with a multinet. Like the name suggests, this is a deployment with multiple nets attached, which allows us to catch plankton at different depths separately. We know that plankton hides for predators in the deep waters during the day and comes to the surface to eat during the night. But how deep do they dive, does this differ between species, and where is their food in the water column? These are all questions we are trying to answer. So far, we encountered many cool species that we have never seen before. We learn something new every day!

Most of the time I spend filtering water for DNA analyses. This is not very exciting and although my lab door is always open, barely anyone pokes their head in. This all changes after a plankton ringnet: the other scientists, as well as the crew come streaming into my lab to see the weird and wonderful world of the plankton. We find lots of small shrimp racing around the petridish, spiky pteropods flapping their huge wings, heteropods with big eyes eating others of their kind. Once I even found a baby squid (that I brought back to the ocean of course). Some pteropods are selected for experiments that focus on their diet and swimming behaviour.

Every water sample that has been taken so far I get part of, which renders my days very busy. They usually start at 3 am in the morning, and processing usually lasts well into the afternoon. Despite the rewarding scientific part of the expedition, there are many things in that will make this time here a special memory. I cherish the sunrises, reading clubs after dinner, the flying fish spotting, and simply being at sea.
I am very thankful for Katja Peijnenburg, who unfortunately couldn’t join the expedition to Cape Verde. She gave me the opportunity to join the RV Pelagia, dive into the life of shelled pteropods, and witnessing their beauty and diversity hands on. The trust from Katja, the interest from the other scientists on board, and the excitement from the crew have been giving me a huge amount of motivation to stay curious and eager to finding out what is happening below the surface.
Friday 24th February, by Yasmine Ourradi
On board of the Pelagia Express!
At the time I am writing this blog only 2 weeks are left before the end of the cruise. Many things are happening and not enough time to process it. We are so happy that we got an extra 40 hours of science, and we are making sure to get the most out of it. It also means working whole days in the lab-container with passable to good music but with great company. We have been analysing pH, carbonates and oxygen samples collected at depths up to 5000 meters with a spectrophotometer. Even if I used this instrument a couple of times before, this has been a learning curve, From, “oh lord how do I do this?” to cries of joy “Yes! We did it!”. If everything goes according to plan, we might also analyse total alkalinity and dissolved inorganic carbon next week on the infamous VINDTA. Thinking of it makes me a bit apprehensive but I’m sure we will be able to do it.

Our days are not only filled with work. The most satisfying part is when we are done in the lab and we take the long stairs to the deck and there, we are faced with an amazing sunset view with the ocean as our constant neighbour. So far, the weather has been very clement, calm ocean and warm days, an ideal combination for a great cruise. The crew members and the rest of the scientists make this experience even more pleasant with a lot of support, fun and group hugs. What else to boost our energy!

On a final note, being surrounded by the ocean makes you think twice about the enormity of our planet. On a map it looks so small, so attainable but the reality is so different.
Tuesday 28 February, by Anne Roozendaal
Now that the week filled with (semi) super stations has come to an end we finally have time to take a breath.. Or do we?? At least there is time to look back on the first two weeks of this cruise and point out some important notes:
- It was a good idea to do a practice station at the start of the cruise because the not-so-well-oiled CTD-sampling machine still had to warm up back then.
- Downloading more than 1 Spotify playlist might have been a good idea.
- Three people in the gym is the absolute maximum, with more people in there the temperature will get absolutely unbearable.
- Do everything Neptune says.

During the first week being in the container to do measurements almost felt like a punishment, partly due to the great weather outside and partly due to the rougher seas we were in back then. But now that we have crossed the equator and the temperatures are way higher we seek coolness in the container and are happy when the measurements can start. To give you an idea of what we do on a superstation-day here is a rough sketch of my day:
- Wake up, watch the sunrise and go to the gym
- Have breakfast
- Write stickers and stick them on the bottles (and make sure that you both fill the boxes the same way so that during sampling you don’t get lost in the boxes)
- CTD sampling! We filled bottles for: oxygen, TA/DIC, pH and carbonate. Chemicals were added to the oxygen samples right away, and then they were stored submerged under water for at least 24 hours until analysis.
- Poisoning the TA/DIC (and pH) samples with mercury chloride to kill all the living things in the bottle so the water will not change anymore until analysis
- Carbonate analysis: filling the cells was done before lunch and after lunch the cells were analyzed in a spectrophotometer, the analysis had to be done within 12 hours after sampling.
- pH analysis, also making use of the spectrophotometer.
- Hopefully be ready on time for reading club
- Science meeting and some more reading club after dinner

Friday 3 March 2023, by Olivier Sulpis (Utrecht University)
It has been two weeks and a half since we have left Cape Town, and we are now a few days away from Cape Verde. In all that time, we have sailed across 50 degrees of latitude, visited many biomes, spotted crowds of dolphins, flying fish and whales. We have sailed over many underwater valleys and mountains, trenches, and seamounts, yet we could see none of them, as all around us was nothing but flat, blue water. The sky and weather evolution has been hard to miss though. The first week, in the summer of the southern hemisphere, was characterized by bright blue skies and warm weather. As we got closer to low latitudes, the air became more humid and the heat harder to bear. Hours after we crossed the equator, we encountered pouring rain, and the next few days were spent covered by gigantic cumulonimbus clouds. We are now back to the northern hemisphere, back to winter.

During all that time, besides the water, plankton, and sediment sampling operations that regularly took place, some unusual science activities took place in one of the Pelagia’s lab. We have been setting up a new kind of experiment meant to simulate the fall of marine snow, which can be thought of as the shower of biogenic material free-falling from the ocean surface to the seafloor.
Most of the marine snow is composed of carcasses, seashells, algae, that sink to the seafloor after the death of organisms living at the ocean surface. There are even some living animals that can be part of marine snow. For instance, there are species of sea snails that cannot swim and spend their whole life attached to air bubbles at the very surface of the ocean. In a moment of inattention, they eventually let go of these bubble rafts and inevitably start sinking to the seafloor. In the ocean, pressure increases about one atmosphere for every 10 meters of water depth. Most human beings would not survive a few tens of meters below the ocean surface. Yet, even at the deep seafloor, most of which is located at depths where pressure is about 500 times that of the atmosphere, life is abundant. The deep ocean is home to a myriad of unknown microbes adapted to those high pressures that feed on marine snow. Only a small fraction of organic marine snow escapes decomposition and becomes up buried in sediments of the seafloor. High pressures of the deep ocean are also causing the dissolution of hard marine snow particles such as seashells.
It is extremely complicated to collect marine snow in situ because abyssal environments are hard to reach, because existing devices to sample marine snow are subject to well-known collection bias, and because retrieved organisms would burst upon depressurization. Instead, we have chosen an alternative approach: simulating the free fall of marine snow particles in the laboratory, using a new reactor in which seawater and marine snow particles collected from a plankton net can be placed and monitored under pressures of up to 500 atmospheres. This allows microbes living in marine snow to do their thing, at the high pressures to which they are acclimated. Pressure within the reactor filled with seawater and marine snow is gradually increased, at a pace that reproduces the marine snow sinking rates of the particles we put in, and we stand there observing what is going on.

Eventually we hope to be able to understand why underwater mountains look pretty much like mountains we have on land: white at the top, covered with marine snow full of white seashells, and darker at the bottom, composed of brown or red clays, with little to no seashells in them. We can see that very well from the sediment cores that we retrieved during this expedition but understanding why that is and what will be the role of all those sediments and marine snowflakes in shaping the future of the Earth will require more effort.
Interpreting all the data we gathered will have to wait, as now is the time for cleaning up the labs, packing up our nets, high-pressure reactors, and other devices, to prepare the ship for the next science expedition. I am grateful to the captain and all the crew for giving me the opportunity to be part of this expedition, for their curiosity and interest, and for always being so helpful when we needed it.

